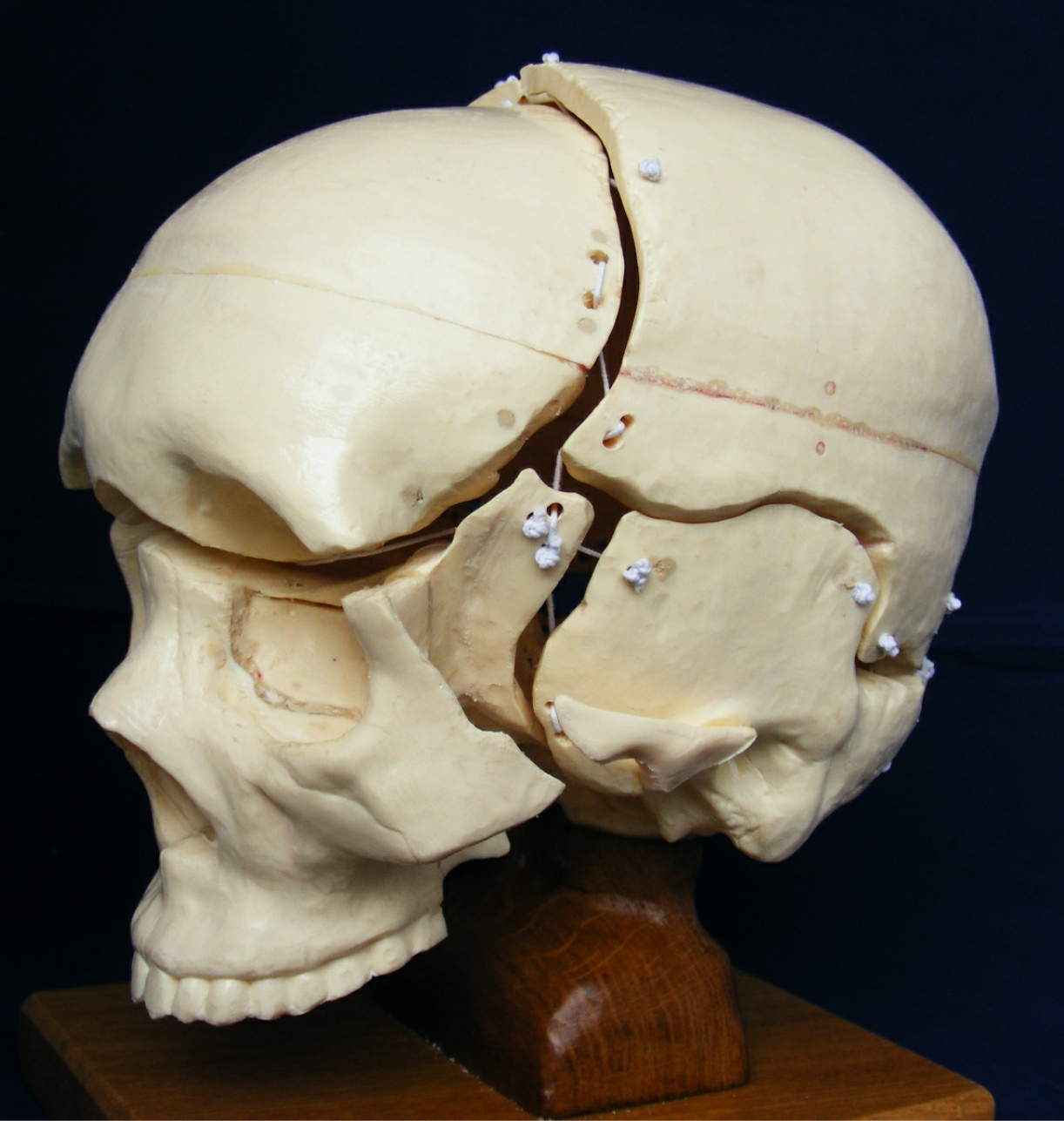
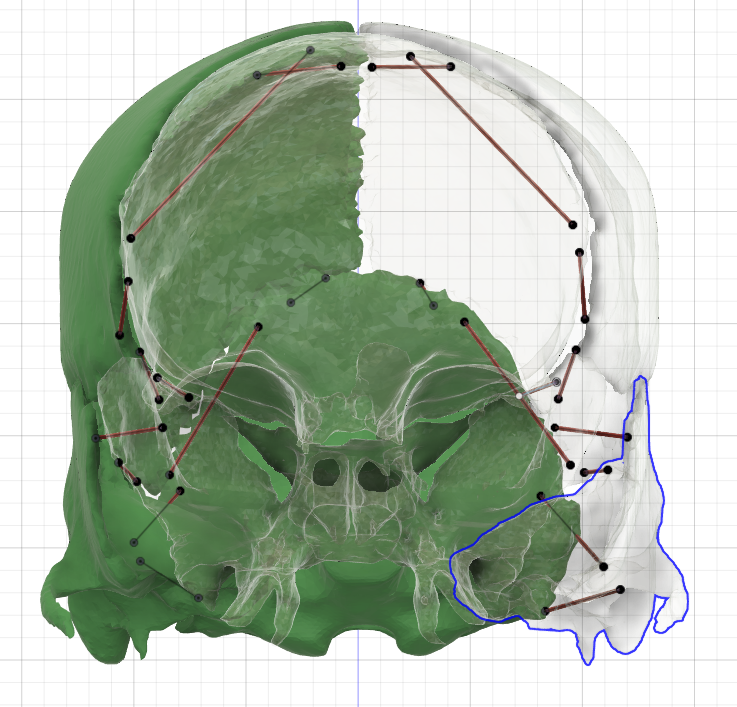
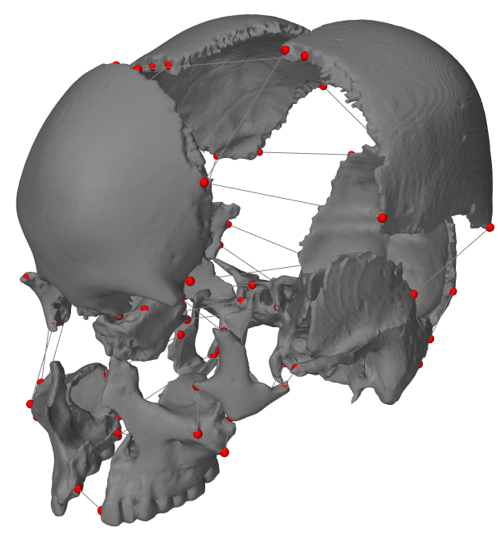
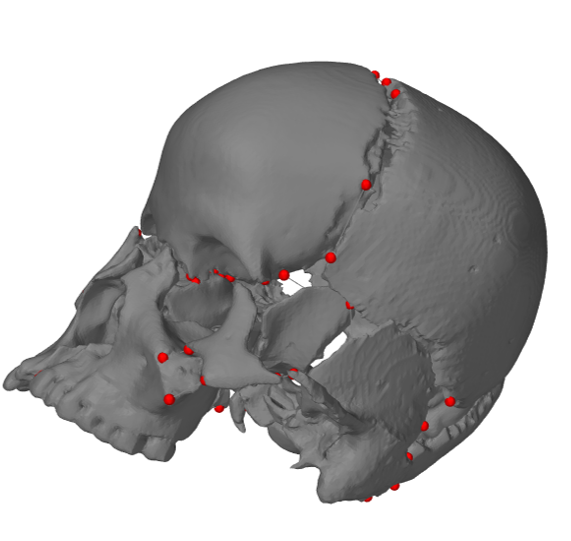
[1] S. Celik, C. Tomruk, D. E. Tanriover, Y. Uyanikgil, O. Bilge, and M. Turgut, "Embryological and Histological Features of the Cranial Sutures," in The Sutures of the Skull: Springer, 2021, pp. 19-42.
[2] J. Libby, A. Marghoub, D. Johnson, R. H. Khonsari, M. J. Fagan, and M. Moazen, "Modelling human skull growth: a validated computational model," Journal of the Royal Society Interface, vol. 14, no. 130, p. 20170202, 2017.
[3] E. G. Takhounts, R. H. Eppinger, J. Q. Campbell, R. E. Tannous, E. D. Power, and L. S. Shook, "On the development of the SIMon finite element head model," SAE Technical Paper, 2003.
[4] J. E. Lloyd, I. Stavness, and S. Fels, "ArtiSynth: A fast interactive biomechanical modeling toolkit combining multibody and finite element simulation," in Soft tissue biomechanical modeling for computer assisted surgery: Springer, 2012, pp. 355-394.
[5] G. Scarr, "A model of the cranial vault as a tensegrity structure, and its significance to normal and abnormal cranial development," International Journal of Osteopathic Medicine, vol. 11, no. 3, pp. 80-89, 2008.
[6] G. Scarr, "Biotensegrity: What is the big deal?," Journal of Bodywork and Movement Therapies, pp. 134-137, 2020.
[7] S. M. Levin, "Tensegrity: the new biomechanics," Textbook of Musculoskeletal Medicine., pp. 150-162, 2015.
[8] C.-M. Boghdady, N. Kalashnikov, S. Mok, L. McCaffrey, and C. Moraes, "Revisiting tissue tensegrity: Biomaterial-based approaches to measure forces across length scales," APL bioengineering, vol. 5, no. 4, p. 041501, 2021.
[9] K. M. Tse, S. P. Lim, V. B. C. Tan, and H. P. Lee, "A review of head injury and finite element head models," Am. J. Eng. Technol. Soc, vol. 1, no. 5, pp. 28-52, 2014.
[10] F. Turquier et al., "Validation study of a 3D finite element head model against experimental data," SAE transactions, pp. 1912-1923, 1996.
[11] H. L. Vu, J. Panchal, E. E. Parker, N. S. Levine, and P. Francel, "The timing of physiologic closure of the metopic suture: a review of 159 patients using reconstructed 3D CT scans of the craniofacial region," Journal of Craniofacial Surgery, vol. 12, no. 6, pp. 527-532, 2001.
[12] E. P. Buchanan, Y. Xue, A. S. Xue, A. Olshinka, and S. Lam, "Multidisciplinary care of craniosynostosis," Journal of Multidisciplinary Healthcare, vol. 10, p. 263, 2017.
[13] D. Johnson and A. O. Wilkie, "Craniosynostosis," European Journal of Human Genetics, vol. 19, no. 4, pp. 369-376, 2011.
[14] M. L. Moss, "Functional anatomy of cranial synostosis," Pediatric Neurosurgery, vol. 1, no. 1, pp. 22-33, 1975.
[15] N. R. Saber et al., "Generation of normative pediatric skull models for use in cranial vault remodeling procedures," Child's Nervous System, vol. 28, no. 3, pp. 405-410, 2012.
[16] J. D. Corrigan, A. W. Selassie, and J. A. L. Orman, "The epidemiology of traumatic brain injury," The Journal of head trauma rehabilitation, vol. 25, no. 2, pp. 72-80, 2010.
[17] Fusion360. (2022). Autodesk Inc. [Online]. Available: https://www.autodesk.ca/en/products/fusion-360/overview
[18] L. Zhang et al., "Recent advances in brain injury research: a new human head model development and validation," Stapp Car Crash Journal, vol. 45, 2001.